Abstract
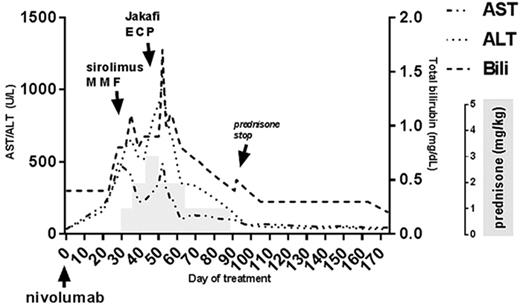
PD-1 inhibition has been shown to effectively treat relapsed Hodgkin's lymphoma after allogeneic stem cell transplant (AlloSCT) by augmenting the graft-versus-lymphoma immune response. While PD-1 blockade post-Allo SCT is associated with a high overall response rate, there is also a high rate of severe graft-versus-host disease (GVHD) following treatment (Blood 2017 130:221-228). Many of the reported fatalities in this patient population have been associated with new onset treatment-refractory GVHD. Interestingly, GVHD occurring in this situation commonly manifests in the liver and can have features of both acute and chronic GVHD. This unique complication raises questions about the most effective treatment strategy. We report the successful management of PD-1 inhibitor-associated liver GVHD in a 31-year-old patient with classic Hodgkin's lymphoma, mixed cellularity subtype, who received the PD-1 inhibitor nivolumab for relapsed disease following a matched sibling donor peripheral blood Allo-SCT. The original indication for Allo-SCT was chemosensitive disease relapse following autologous stem cell transplant. The conditioning regimen was reduced-intensity busulfan and fludarabine and GVHD prophylaxis was low dose methotrexate and tacrolimus. Approximately 180 days after Allo-SCT the patient developed extensive chronic GVHD (elevated liver transaminases and decreased FEV1) which was successfully treated with a short course of oral steroids. Positron emission tomography-computed tomography (PET-CT) scan 1 year after Allo-SCT showed recurrent disease above and below the diaphragm. Brentuximab was initiated, resulting in resolution of hypermetabolic lymphadenopathy, but treatment was stopped after the third dose due to a severe infusion reaction. Three months later, PET-CT revealed multiple foci of high metabolic activity suggesting relapse. Vorinostat and sirolimus were initiated, and remission was obtained for the third time after Allo-SCT while on this regimen. After five months of vorinostat and sirolimus, the patient developed a pulmonary infection and subsequent biopsy-proven disease relapse. The patient was started on single agent nivolumab 3 mg/kg. Approximately 30 days after infusion the patient developed painful sclerodermatous lesions on the shins and increasing liver transaminases (Figure 1). Further treatment with nivolumab was held and 1 mg/kg/day of prednisone-equivalent was initiated. After 1 week, no improvement in liver enzymes was observed and prednisone was increased to 2 mg/kg/day. Sirolimus and mycophenolate mofetil were added. Despite initial improvement, transaminases and bilirubin increased again and a liver biopsy on day 48 of therapy showed lymphocytic cholangitis, cholestasis, ductopenia, and portal-to-portal bridging fibrosis consistent with chronic GVHD. AST peaked at 488 U/L, ALT peaked at 1093 U/L, and total bilirubin peaked at 1.7 mg/dL. Prednisone was increased to 3 mg/kg/day and extracorporeal photopheresis and Jakafi were initiated. Liver transaminases steadily declined. Prednisone was slowly tapered and maintenance therapy with Jakafi, sirolimus, and monthly extracorporeal photopheresis has maintained remission without a subsequent GVHD flare. PET/CT six months after nivolumab showed no evidence of disease, and the patient remains in clinical remission more than eight months after treatment. This case study highlights several uncertainties in the management of GVHD following PD-1 blockade. Should patients receive higher doses of steroids upfront (> 1 mg/kg/day)? Should GVHD prophylaxis be used? How should second-line therapies be chosen and how quickly should they be added? The largest case series to date by Haverkos et al. reports a high rate of treatment resistant GVHD suggesting that rapid escalation of immunosuppression is needed. Overall, this case represents a successful management of a newly-recognized and severe form of GVHD that can occur following PD-1 blockade in Allo-SCT recipients. Given that nivolumab is one of the few effective treatment options for relapsed Hodgkin's lymphoma after Allo-SCT, effective strategies for managing GVHD after PD-1 inhibition are needed.
Lin: Jazz Pharmaceuticals: Consultancy. Ganguly: Amgen: Other: Advisory Board; Seattle Genetics: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal